南湖新闻网讯(通讯员 任泽恒)近日,我校动物科学技术学院、动物医学院赵凌教授团队研究成果以“A SynB1-conjugated antibody cocktail crosses the blood–brain barrier to produce a therapeutic effect on rabies”为题在PNAS发表。
狂犬病是一种造成宿主中枢神经系统感染的重要人兽共患病,目前分布于全球150余个国家,每年死亡人数超过6.9万人。狂犬病的最大特征是发病后不可治愈,死亡率接近100%。 本研究提出了一种狂犬病治疗新策略,在狂犬病症状初期展现出有效的治疗效果。该策略通过基因工程技术将狂犬病毒中和抗体Fc段偶联了一个血脑屏障(BBB)穿膜肽SynB1,能够显著提升抗体通过BBB并进入大脑的能力,从而提高狂犬病的治疗效果,并为治疗除狂犬病毒以外的其他嗜神经病毒感染提供了重要借鉴。
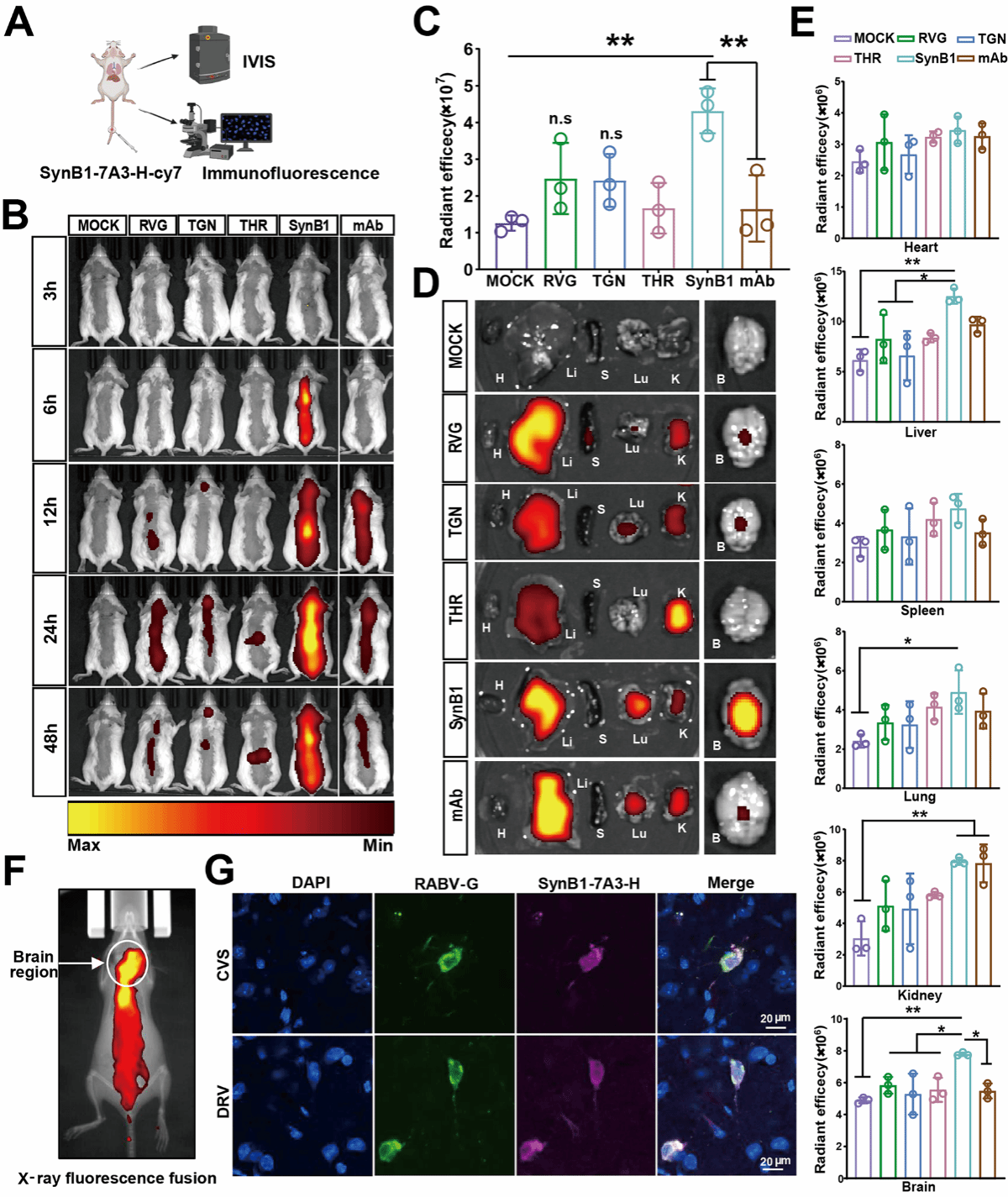
中和抗体与穿透肽偶联后的入脑效率检测
该研究首先构建了四种保留了对狂犬病毒具有中和效力的人鼠嵌合抗体。为了实现抗体脑靶向递送,作者将一种先导嵌合抗体(7A3-H)与4种不同的具有潜在穿越BBB能力的细胞穿膜肽偶联。体内荧光成像结果显示,在穿越BBB效率方面,SynB1偶联抗体显著优于其他肽的偶联抗体,从而更有效地将抗体递送至小鼠脑部。作者将分别靶向狂犬病毒G蛋白II/III/IV表位的三种抗体混合物与SynB1偶联,在感染致死性剂量狂犬病毒5天后给药,可使小鼠存活率达到80%。上述结果表明,SynB1介导的血脑屏障穿越显著提升了中和抗体进入大脑的能力,从而实现了早期狂犬病临床症状的有效治疗。这种抗体-穿膜肽偶联策略为探索狂犬病临床治疗提供了一个新概念,后续将具有更高中和活性的人源抗体与高效靶向血脑屏障的肽偶联,进一步提高该策略的可行性。
开云电竞游戏动物科学技术学院、动物医学院博士生任泽恒、王才茜为论文的共同第一作者,赵凌教授和周明副教授为论文的通讯作者,开云电竞游戏为通讯作者单位。该研究得到了国家重点研发计划项目的资助。
论文链接:https://www.pnas.org/doi/10.1073/pnas.2516465122
【英文摘要】
Rabies, caused by the neurotropic lyssavirus rabies virus (RABV), is nearly 100% fatal after symptom onset. A fundamental challenge for rabies therapy is that the blood–brain barrier (BBB) prevents peripherally administered neutralizing antibodies from entering the central nervous system (CNS) to clear the infection. In this study, we initially generated four human‒mouse chimeric antibodies with preserved neutralizing potency against RABV to increase its translational potential. To achieve brain antibody delivery, we conjugated a lead chimeric antibody (7A3-H) to different cell-penetrating peptides with potential BBB-penetrating capacity (RVG, TGN, THR, and SynB1). In vivo fluorescence imaging revealed that SynB1 conjugation significantly outperformed conjugation with the other peptides in terms of BBB penetration for brain antibody delivery. Furthermore, a triple-antibody cocktail (targeting epitopes II/III/IV of RABV-G) conjugated to SynB1 resulted in 80% survival in mice infected with lethal CVS or DRV strains when it was administered at 5 days post-infection (when CNS invasion and symptoms were evident). In contrast, the unconjugated cocktail provided only 20% (CVS) or 0% (DRV) survival. Together, these data demonstrated that SynB1-mediated BBB penetration dramatically improved the therapeutic efficacy of anti-RABV antibodies, enabling postsymptom rescue of rabies. This antibody‒peptide conjugation strategy provides a proof of concept for advancing rabies therapy, specifically by leveraging peptide-mediated enhancement of blood-brain barrier (BBB) penetration.
审核人 赵凌